Using these values we will draw the nucleus of the Lithium atom. A step-by-step explanation of how to draw the Li2S Lewis Dot StructureFor Li2S we have an ionic compound and we need to take that into account when we draw.

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit
Find the Number of Electrons Needed to Make the Atoms Happy.

. Once we know how many valence electrons there are in Lithi. If you have access to a periodic table open it now. For carbon there are four valence electrons two in the 2s subshell and two in the 2p subshell.
H He Li Be B C N. Draw the electron dot diagram for neutral lithium. Create a neutral hydrogen atom.
Li with two dots on the top and then one on the right for Lithium. Use the Gizmo and your periodic table to find atomic number for the number of protons and electrons and atomic mass to calculate the number of neutrons in order to create a neutral atom. Use the Gizmo to create a neutral atom of each of the following elements.
Therefore for the Lithium atom also. The electron configuration of Li is 1s22s1. A beryllium atom with two valence electrons would have the electron dot diagram below.
Lithium nitride Li3N NLi3 is an ionic compoundThree lithium atoms lose one electron each they each become 1 chargeOne nitrogen atom gains those three e. Since electrons repel each other the dots for a given atom are distributed evenly around the symbol before they are. Again it does not matter on which sides of the symbol the electron dots are positioned.
The Bohr model of Lithium is drawn with only two electron shells the first shell contains 2 electrons and the second shell contains only 1 electron. Turn off Show electron dot diagram. These instructions outline the Kelter strategy to draw Lewis structures for molecules.
Turn off Show electron dot diagram. The periodic table Get the Gizmo ready. Draw an electron dot diagram for each.
Find the Total Number of Valence Electrons. Use the Gizmo to create a neutral atom of each of the following elements. Determine the number of bonds in the molecule.
The valence electrons of an atom are shown in an electron dot diagram. Choose a Central Atom. Turn off Show electron dot diagram.
Each dot represents a valence electron. The electron configuration of Li is 1s22s1. When you are finished check your answers.
Actually the number of electrons as well as protons in an atom is the same as they both are equal to the atomic number of the atom. Now we will calculate the number of electrons for the Lithium atom. Draw the electron dot diagram for neutral lithium.
Turn off Show electron dot diagram. In the above diagram p protons n neutrons. Draw the electron dot diagram for neutral lithium.
Use the Gizmo to create a neutral atom of each of the following elements. The electron dot structure for lithium is Li the element symbol with one dot on the upper right side. 1 Draw the electron dot diagram for neutral lithium.
The valence electrons of an atom are shown in an electron dot diagram. Each dot represents a valence electron. When you are finished turn on Show electron dot.
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol. Draw an electron dot diagram for each. A step-by-step explanation of how to draw the Li2O Lewis Dot StructureFor Li2O we have an ionic compound and we need to take that into account when we draw.
How do you draw the Lewis dot diagram for lithium and fluorine. So you can see that there are 2 inner electrons in the 1s sublevel. For the Li structure use the periodic table to find the total number of valence electrons for Li.
A step-by-step explanation of how to draw the LiCl Lewis Dot StructureFor LiCl we have an ionic compound and we need to take that into account when we draw. However conventionally we draw the dots for the two p electrons on different sides. Lithium is neutral and its atomic number is 3 hence the number of protons and electrons available for its Bohr diagram is also 3.
When you are finished turn on Show electron dot diagram and check your answers. Draw an electron dot diagram for each. How do you draw a electron dot diagram for magnessium oxide.
As usual we will draw two dots together on one side to represent the 2s electrons. A neutral atom has equal numbers of protons and electrons so a neutral Li atom also has 3 electrons. There is a faster way to do it than building all of the atoms if you know your Periodic.

Lewis Dot Structure For Lithium Li Youtube

Lewis Dot Structure For Lithium Li Youtube

4 Diagram Turn On Show Electron Dot Diagram The Valence Electrons Of An Atom Are Course Hero

File Lewis Dot Li Svg Wikimedia Commons

Bohr Rutherford Lewis Dot Diagrams Ppt Download
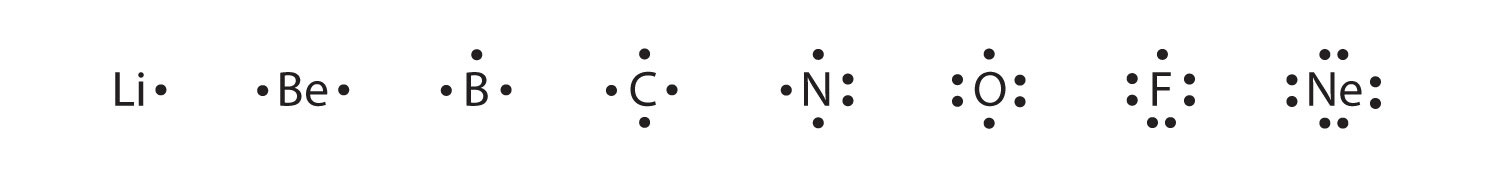

0 comments
Post a Comment